
Blog Post #9 – In previous blog posts, we discussed how to identify confined spaces, and provided several examples of such spaces. Next we will examine the factors that determine whether or not each confined space identified at your workplace must be classified as a permit-required confined space, starting with a hazardous atmosphere that contains a flammable gas, vapor, or mist in excess of 10% of its lower flammable limit, or LFL.
OSHA Definition of a Permit-required Confined Space
The Federal OSHA definition of a permit-required confined space for general industry can be found in 1910.146, paragraph B, titled definitions, and in 1926.1202, also titled definitions, for the construction industry. That is where OSHA lists the four types of hazards that require a confined space be classified as a permit required confined space. Those four types of hazards are:
- The space contains an actual or potential hazardous atmosphere;
- The space contains a material with the potential to engulf the entrant;
- The space is configured to trap or asphyxiate the entrant; or;
- The space contains some other recognized serious safety or health hazard.
What is a Hazardous Atmosphere?
The Federal OSHA definition for “hazardous atmosphere”, the first classification listed above, is also found in 1910.146, paragraph B, definitions, for general industry, and in 1926.1202, definitions, for the construction industry.
There are five different types, or classifications, of hazardous atmospheres, listed in these two OSHA definitions. Those classifications, which are nearly identical in the two standards, are:
(1) – Flammable gas, vapor, or mist in excess of 10 percent of its lower flammable limit (LFL);
(2) – Airborne combustible dust at a concentration that meets or exceeds its LFL;
(3) – Atmospheric oxygen concentration below 19.5 percent or above 23.5 percent;
(4) – Atmospheric concentration of any substance for which a dose or a permissible exposure limit is published in subpart G, Occupational Health and Environmental Control, or in subpart Z, Toxic and Hazardous Substances, of this part and which could result in employee exposure in excess of its dose or permissible exposure limit;
(5) – Any other atmospheric condition that is immediately dangerous to life or health.
The balance of this particular blog post focuses on the first category of a hazardous atmosphere listed above, which is a flammable gas, vapor, or mist greater than 10% of its lower flammable
Greater Than 10 Percent Lower Flammable Limit (LFL)
OSHA includes this type hazard because they do not want employers to wait until conditions are ripe for a flash fire or explosion to occur inside of a confined space before taking precautionary measures. Atmospheric conditions inside a confined space that contain a low level of flammable gas, vapor, or mist could quickly change for the worse. Therefore, OSHA regulations classify a flammable gas, mist, or vapor present inside a confined space at levels over 10% of its LFL as a hazardous atmosphere. Once that level is reached inside a confined space, or if there is a reasonable probability that it will develop within the space during entry operations, that space must be classified as a permit-required confined space.
Lower Flammable Limit (LFL) Explained
So, what is LFL? It stands for Lower Flammable Limit. The LFL is the minimum amount of a particular flammable gas, vapor, or mist that must be present in the atmosphere to ignite and burn. If you introduce an extremely small amount of a flammable gas, vapor, or mist into the atmosphere of a confined space, it might not ignite when exposed to an ignition source (such as a spark or flame) because there is not enough of the material present to form a flammable mixture in the air. How much flammable gas, vapor, or mist is needed in the atmosphere for a fire or explosion to occur? That depends on the gas, vapor, or mist present inside either space, as they all have a different LFL.
What is LEL?
Be aware the term “LFL” is also referred to as “LEL”, or Lower Explosive Limit. The terms LFL and LEL are basically interchangeable. While OSHA uses LFL in their confined space standards, LEL is commonly used by many gas detection equipment manufacturers, as well as in many written confined space entry programs. Also, I may on occasion use the term LEL throughout future blog posts.
LFLs of Common Flammable Gasses
The LELs of a few common combustible materials are listed in the chart below. An LEL is expressed as a percentage of the atmosphere within the confined space that is comprised of a particular combustible gas, vapor, or mist. For example, the LEL of Methane gas, with the chemical identifier of CH4, is 5% of the atmosphere at any given point. As you can see, the LELs of various gases differ quite a bit.
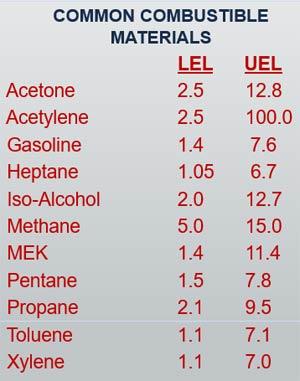
What does UFL mean?
Note that each of the combustible materials listed in the chart also have their own UFL, or Upper Flammable Limit (expressed in the chart as an UEL). If a flammable gas, vapor, or mist is present in the atmosphere in quantities greater than its UFL, it will not ignite and burn. I bring up this term because while OSHA does not regulate UFLs, they do come into play when we discuss gas detection equipment in later blog posts.
Flammability Characteristics of Methane Gas
As mentioned earlier, the LFL of Methane gas is 5%. So, if Methane gas is present in a confined space, but it does not make up at least 5% of the atmosphere at any point within the space, there will be no flash fire or explosion if there is a spark or other source of ignition. And the upper flammable limit, or UFL, of Methane gas is 15%; So, a confined space containing higher levels of Methane would not burn or explode either. However, if the amount of Methane gas increases or decreases to fall somewhere between its LFL and UFL at any point inside a confined space and there is any source of ignition in that same area, a fire or explosion will occur.
Since Methane gas has an LFL of 5% of the atmosphere, a hazardous atmosphere as defined by OSHA would be present inside a confined space if the amount of Methane gas in the atmosphere exceeds 10% of that level. The presence of that level of Methane gas, which equates to just 0.5% of the total atmosphere, means the space would have to be classified as a permit-required confined space.
Other Hazards Associated with Methane Gas
Before moving on, let’s take a moment to discuss Methane gas, or CH4, a little more, as this is a hazardous gas commonly found in a variety of confined spaces. Methane is a colorless, tasteless gas that has no odor, so its presence cannot be detected by your senses alone. But too much Methane gas inside of a confined space creates not only a potentially flammable atmosphere, as previously discussed, it can also displace oxygen in the space, creating a deficiency of oxygen for an entrant to breathe. Fortunately, while Methane is a highly flammable gas that can also displace oxygen, it is one of the few flammable gases that is not toxic.
Where is Methane Gas Commonly Found?
Methane is created when organic matter decomposes. Therefore, it is commonly found in potentially harmful quantities within confined spaces such as sewers and related facilities, manure pits, and in silos that have held grain or similar materials that has rotted. Methane is also commonly found in confined spaces such as underground utility vaults, especially those located near landfills (the rotting trash creates Methane). Therefore, these spaces are typically classified as permit-required confined spaces.
More Examples of Confined Spaces With Potentially Flammable Atmospheres
Other examples of confined spaces with a reasonable potential to contain an atmosphere containing a flammable gas, vapor, or mist above 10% of its LFL include pipelines, tanks and other vessels that have contained fuels or other flammable or combustible materials, even when they have been emptied of their contents. Also, confined spaces where work processes such as cutting with an oxy-acetylene torch or painting with flammable paints and solvents is taking place inside could potentially contain a hazardous atmosphere. And confined spaces such as vaults or tunnels in which there are leaking valves or piping used to transport flammable gas could also contain a hazardous atmosphere. As such, each of these spaces would also likely be classified as a permit-required confined space.
So, in review, any confined space with an actual flammable gas, vapor, or mist present at levels greater than 10 percent of its lower flammable limit, or LFL, is considered to have a hazardous atmosphere. And that means the space must be classified as a permit-required confined space. The same is true for any confined space in which that level of a flammable atmosphere could reasonably be expected to develop during entry operations. You may also hear the term LEL, which stands for lower explosive level, being used to describe a hazardous flammable atmosphere inside a confined space, as that term is synonymous with LFL.
In our next blog post (#10), we will examine the second criteria of a hazardous atmosphere that could be present inside of a confined space (combustible dust). In the meantime, please provide your feedback and questions to this blog post in the comments section below. And as always, I urge you to share a link to this confined space training blog post with anyone in your network who could benefit from this information. Thanks – Curtis
Why is Methane the preferred gas to calibrate gas monitors?
I have come across Pentane being used in a specific industry which is underground fuel storage tanks (aviation fuel)
Hello Paul. Very good question. Manufacturers of gas detection meters all recommend to calibrate their instruments’ LFL sensors with the same gas that will be in the space, if possible, or with one close in properties to the same gas if an exact match is not possible. Since so many meters are set up to detect flammable gas (Methane) in sewers and other underground installations, Methane is preferred as a calibration gas, as it occurs naturally in those environments. So more users default to that gas for calibration. However, people entering aviation fuel tanks are usually better off using Pentane, as they get a more accurate LFL readout of the percentage of gas present in those spaces. Hope that helps explain clearly, if not let me know and I’ll try to do a better job. Thanks,
Great information, what is your viewpoint on inerting vs ventilating flammable atmospheres in confined spaces for general industry?
Is inerting required anytime a flammable substance is present e.g. hydrogen sulfide. Or can explosion proof blowers be sufficient for controlling the potential flammable atmosphere.
Hello Jared. Sorry for the delayed reply, been dealing with the pre and post effects of Hurricane Milton. This is an excellent question, and one that we will certainly expand upon when we post to the blog about controling atmospheric hazards.
Inerting is not always required when a flammable substance like hydrogen sulfide (H₂S) is present, but it can be one of the controls used to prevent the formation of a flammable atmosphere. The choice between inerting and using explosion-proof blowers depends on the specific circumstances, including the concentration of the flammable substance, the type of work being performed, and the applicable safety regulations.
Inerting, which is typically done by adding an inert gas such as nitrogen or carbon dioxide into a confined space in a quantity sufficient to reduce the oxygen concentration below the level required to sustain combustion, is most often used when there is a high risk of ignition or when the concentration of the flammable substance may fluctuate near its explosive limits. Inerting is commonly applied in situations where it is difficult to guarantee that the flammable substance will stay below its lower explosive limit (LEL), or where the environment is enclosed or pressurized. It can also be used as a more robust safeguard in high-risk operations.
Inerting provides a relatively high level of control over the atmosphere, eliminating the risk of ignition by displacing oxygen and ensuring that a flammable environment cannot form.
On the other hand, explosion-proof blowers can be sufficient to control the flammable atmosphere when they are sufficient to ensure that the concentration of the flammable substance (such as H₂S) remains below its lower explosive limit (LEL). Ventilation with explosion-proof equipment helps dilute the concentration of the gas to safe levels (below 10% of its LEL), preventing the formation of a combustible atmosphere. This method is often used in confined spaces or areas where flammable gases may accumulate but can be adequately ventilated. Continuous monitoring is usually required to ensure that the atmosphere remains below 10% of its LEL. Explosion-proof blowers are less disruptive to operations compared to inerting and are generally easier to set up.
Considerations:
1. Risk assessment: A thorough risk assessment should be performed to evaluate the concentration of the flammable gas, the potential for ignition sources, and the ability to maintain safe concentrations with ventilation alone.
2. Regulatory requirements: Local and industry-specific regulations may dictate the necessity of inerting in certain operations involving flammable substances. For example, in some situations, inerting may be a regulatory requirement regardless of other controls.
3. Monitoring: Continuous gas detection is essential, whether you’re using explosion-proof blowers or inerting, to ensure that gas concentrations remain within safe levels.
In summary, while explosion-proof blowers can be a sufficient control in some situations, inerting may be required in others, particularly in higher-risk environments where additional safety measures are needed. Hope this helps.
Thank you for all of the great feedback!
You bet!