Blog Post #12 – In this post to The Confined Space Training Blog, we will examine the fourth category listed in the Federal OSHA definition for a hazardous atmosphere, which is an atmosphere that contains any toxic substance present in quantity greater than its permissible exposure limit, or PEL.
Before moving on, make certain to pay close attention to an important footnote to this definition in the OSHA definition section for this standard. OSHA states that this category of hazardous atmosphere only applies “if the topic substance can cause death, incapacitation, impairment in ability to self-rescue, injury, or acute illness due to its health effects.”
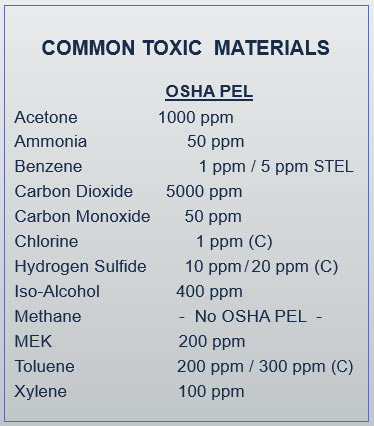
For example, exposure to ammonia in the atmosphere at levels above its PEL for an extended period of time would cause the entrant to experience burning eyes and throat, difficulty breathing, and make it extremely difficult to function normally; exposure to extremely high levels could even lead to death. This condition would meet the definition of a hazardous toxic atmosphere in the confined space standards. However, exposure to silica or asbestos at levels over their respective PELs, although harmful in the long term, would typically not cause any immediate reaction. So, overexposure to those particular substances would not meet the definition of a hazardous atmosphere as defined in the OSHA confined space standard (although normal protective measures required by other OSHA standards would still be applicable).
What are OSHA PELs?
The term PEL represents the maximum exposure level for a particular contaminant that an employee may be exposed to at work, averaged over a specified period of time, without suffering negative health effects or needing to take protective measures such as use of a respirator. PELs for various contaminants are established by Federal OSHA and are listed in different parts of the OSHA code of federal regulations.
Part 1910 lists the PELs that apply to general industry worksites. Part 1926 for construction and Part 1915 for shipyards also have PELs, and some of those may differ from those listed in Part 1910. In addition, many states operate their own OSHA approved state program. Some have adopted the federal OSHA PELs, but a few states have established their own PELs, some which are lower than federal OSHA’s.
In addition to the mandatory OSHA PELs, there are some other voluntary limits that you may hear mentioned. For example, The American Conference of Governmental Industrial Hygienists, or ACGIH, has developed threshold limit values, or TLVs, for many substances. And The National Institutes of Occupational Safety and Health, or NIOSH, developed recommended exposure limits, or RELs, for many substances. These are based on more recent research than the OSHA PELs in many cases, and may be set lower (or higher) than the OSHA PEL for some substances. Employers MAY voluntarily choose to utilize these lower limits instead. Also, these voluntary limits are often utilized when OSHA has not established a mandatory PEL for a specific substance.
The permissible exposure limits for most individual substances in gas and vapor form are presented as parts per million, or PPM, which is a way to measure very small quantities of toxic air contaminants at a molecular level. The more toxic the substance, the lower the PEL. For example, the 1910 General Industry permissible exposure limit for ammonia is 50 ppm.
To help visualize the concept of substances being measured in parts per million, or ppm, consider this; if a contaminant evenly dispersed in a space makes up one half of the total atmosphere in that space, it would be measured at 500,000 ppm. If the substance made up 5% of the total atmosphere, it would be measured at 50,000 ppm, and if a substance made up 1% of the total atmosphere, it would measure at 10,000 ppm. A substance making up one tenth of 1% of the total atmosphere would measure at 1,000 ppm, and a substance making up one one-hundred-thousandth of a percent would be measured at 10 ppm. And a few substances are so toxic, their permissible exposure limits are actually measured in parts per billion, or PPB.
The table below shows the federal OSHA PELs for a few common toxic substances found in some confined spaces.
Different Types of OSHA PELs
Unless noted otherwise, OSHA permissible exposure limits are based on an “eight-hour time-weighted average,” also known as TWA. 8-hour TWA means that the employee exposure to the substance is averaged over an eight-hour work shift. For example, the OSHA PEL for acetone is listed as 1,000 ppm, which is averaged over 8 hours.
Some permissible exposure limits are appended with a designation of ST or STEL, which stands for “short term exposure limit.” This is an exposure limit that is averaged over a shorter, 15-minute time period, as opposed to the 8-hour period. For example, the PELs for benzene are listed as 1 ppm based on an 8-hour TWA, but a STEL of only 5 ppm.
A few other substances are marked with the letter C, which stands for ceiling limit. For example, the PEL for chlorine is a ceiling limit of only 1 ppm. This limit is instantly reached any time the employee is exposed to the substance at the level specified.
It is important for employers to identify the toxic substances that employees may be exposed to when entering confined spaces. It is also important for entrants and attendants to know about the modes of exposure, as well as the signs and symptoms of overexposure, to toxic substances. This information can usually be obtained from each substance’s safety data sheet, or SDS.
Toxic Atmospheres in Confined Spaces
So, in review, a hazardous atmosphere exists where a toxic substance is present in a quantity greater than its permissible exposure limit (PEL). This can occur in confined spaces such as, but not limited to, tanks and vessels where a toxic product has been stored or is being utilized, and in underground spaces, such as crawl spaces, utility vaults and sewers, where a hazardous substance such as hydrogen sulfide (H2S) can migrate into the space.
Also, toxic gases such as CO can be created by equipment such as generators, chop saws, and compactors that are powered by internal combustion engines. As a reminder, if there is a hazardous substance for which OSHA has not established a PEL, refer to that substance’s safety data sheet, or SDS, for exposure recommendations and guidelines.
In our next blog post (#13), we will examine the fifth and final criteria of a hazardous atmosphere that could be present inside of a confined space (any other IDLH atmosphere). In the meantime, please provide your feedback to this blog post about toxic atmospheres in the comments section below.
And as always, I hope you will take a moment to spread the word about our confined space training blog by sharing a link to this post with others in your network so they can benefit from this information. Thanks – Curtis