
Blog Post #11 – In this post to The Confined Space Training Blog, we will examine the third category listed in the Federal OSHA definition for a hazardous atmosphere, which is an atmospheric concentration of oxygen less than 19.5%, or greater than 23.5%.
Atmospheric Composition
Oxygen is critical to support human life. However, under normal conditions, over 78 percent of the air we breathe is actually comprised of nitrogen, which is an inert gas. And oxygen, commonly referred to as O2, makes up approximately 20.9% of the atmosphere.
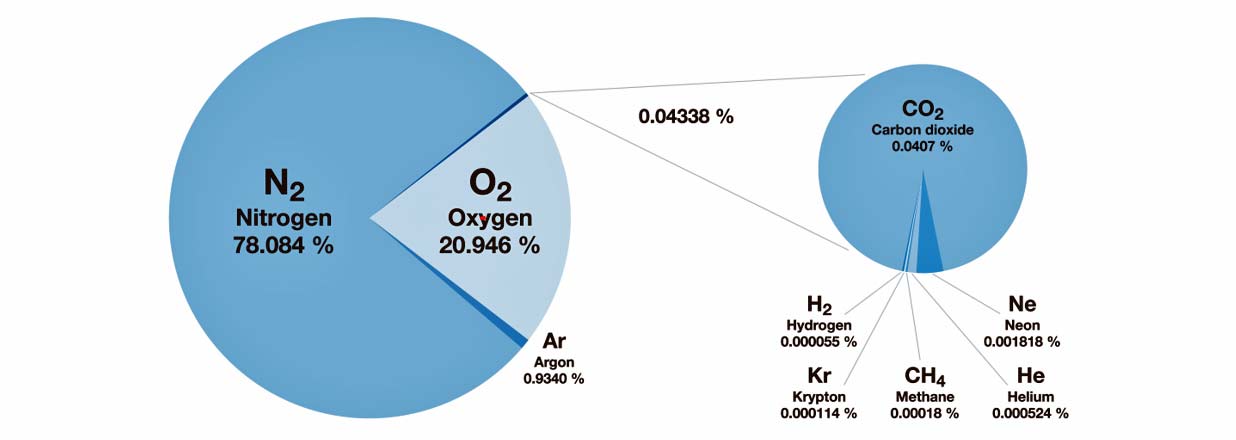
If oxygen levels were to fluctuate slightly, there would most likely be no ill effects on a healthy person. However, a greater decrease or increase in oxygen levels could prove to be harmful; even deadly.
So, OSHA sets the threshold for an oxygen deficient atmosphere at anything less than 19.5% oxygen, and the threshold for an oxygen enriched atmosphere is reached when the O2 levels exceed 23.5%.
What Can Cause an Oxygen Deficient Atmosphere?
One way an atmosphere may become oxygen deficient is if the oxygen gets consumed. One cause of oxygen consumption is flames. A flame must have adequate oxygen to burn. As the flame burns, the oxygen is consumed. That is why the flame on a small candle will slowly extinguish if you cover the candle with a glass, preventing it from getting more oxygen. Similarly, a poorly ventilated confined space in which there has been a flash fire, or where flame producing equipment is present, could become oxygen deficient.
Oxidation is another cause of oxygen deficiency. When metal rusts, that process, called oxidation, consumes oxygen. So does the process of concrete curing. Therefore, confined spaces with a rusty interior surface, or confined spaces formed with freshly poured concrete, could also become oxygen deficient over time.
An oxygen deficient atmosphere can also be created by displacement of the atmosphere, which can occur if another gas fills up the confined space and supplants the oxygen. This happens inside sewers and underground vaults quite often, when naturally-occurring Methane gas seeps into the space. Another way for this to occur is when another gas is accidentally introduced into a confined space through a leaking pipe, cracked hose, or partially open valve. For example, a leaking hose containing Argon or other inert shield gases used during some welding processes is one potential cause of oxygen displacement inside a confined space.
In relatively rare cases, an inert gas is introduced into a confined space purposely, to displace oxygen. This can help prevent materials inside the space from oxidizing. Purging the space with an inert gas can also prevent a highly-flammable atmosphere from igniting during entry operations.
Another way oxygen can be displaced is when certain chemicals are mixed inside of a confined space. For example, mixing vinegar and baking soda creates a bubbly concoction that releases carbon dioxide gas when the bubbles burst. When these or other reactive chemicals are present in great enough quantities, the resulting chemical reactions could release gases that eventually build up to levels high enough to displace oxygen in the confined space.
A less common cause of oxygen deficiency is adsorption. Some manufacturing processes utilize activated charcoal filters to capture unwanted gases. Sometimes a portion of the oxygen adheres to the activated charcoal, which can result in lower oxygen levels in the air.
Effects of Oxygen Deficiency
The effects of oxygen deficiency on a person vary as the level of oxygen drops. In reality, most people in good health would probably not perceive much, if any, ill effect when oxygen levels drop to 19.5%, the threshold of oxygen deficiency. However, when oxygen levels drop to around 16%, the entrant may start suffering the symptoms of hypoxia, where they find it harder to breathe and start to become nauseous and drowsy. When oxygen levels drop to 12% or less, the entrant will become unconscious, and when oxygen levels drop to 6% or less, brain cells begin to deteriorate, and death occurs through asphyxiation.
What is an Oxygen Enriched Atmosphere?
When oxygen levels inside a confined space exceed 23.5% by volume, an oxygen enriched atmosphere is present. One common reason this occurs is because pure oxygen is introduced into the space, either accidentally or on purpose.
Leaking hoses, pipes, or valves on equipment that utilize pure oxygen can result in an oxygen enriched atmosphere inside a confined space. That is why the OSHA welding and cutting standards require an oxy-fuel cutting torch be removed from inside a tank or other enclosed space when not in use.
In other cases, pure oxygen is used to ventilate a confined space with an oxygen deficient atmosphere. This should never be done, as the result can be the introduction of too much oxygen into the confined space. To control an oxygen deficient confined space, blow fresh air into the space using a ventilation fan or blower stationed outside the space. And never place oxygen cylinders, or any other gas cylinders, inside of a confined space, as their contents could leak into the space and adversely affect oxygen levels.
Effects of Oxygen Enrichment
So, why is too much oxygen a bad thing? Primarily, it is because oxygen accelerates the combustion process. In an oxygen enriched atmosphere, the oxygen can saturate the worker’s clothing or other combustible materials inside the space. Then, a single spark from a grinder, torch, or even static electricity can ignite the oxygen-saturated items, which will cause them to burn rapidly.
OSHA Requirements for Oxygen Levels in a Confined Space
In review, an oxygen deficient atmosphere occurs when oxygen levels drop below 19.5% of the atmosphere by volume, and an oxygen enriched atmosphere occurs when oxygen levels exceed 23.5% of the atmosphere by volume.
Oxygen deficiency can occur in many confined spaces, including, but not limited to, tanks and similar vessels that have contained a product whose vapors could displace the oxygen, or that are subject to having the inside purged with an inert gas such as nitrogen. These conditions could also occur inside sewers and underground utility vaults where methane or other naturally occurring gases could collect and displace the oxygen. And oxygen enrichment can occur inside confined spaces into which oxygen accidentally leaks, or where it is introduced into the space on purpose.
In our next blog post (#12), we will examine the fourth criteria of a hazardous atmosphere that could be present inside of a confined space (a toxic atmosphere). In the meantime, please provide your feedback to this blog post about oxygen deficient and oxygen enriched atmospheres in the comments section below.
And as always, I hope you will take a moment to spread the word about our confined space training blog by sharing a link to this post with others in your network so they can benefit from this information. Thanks – Curtis